Zinc Chloride Solution Manufacturer and Supplier
CAS No.: 7733-02-0 | EC Number.: 231-793-3 | Molecular formula.: ZnSO4, O4SZn
Zinc Chloride Solution
Zinc Chloride Solution Manufacturers, Zinc Chloride Solution Suppliers, Zinc Chloride Solution Formula, Zinc Chloride Solution MSDS, Zinc Chloride Solution SDS, Zinc Chloride Solution Exporter in Mumbai, India.
Vinipul Chemicals Pvt. Ltd. is a leading manufacturer, supplier, and exporter of high-purity Zinc Chloride Solution (CAS No. 7646-85-7) in India. As a leading zinc chloride manufacturer, we are known for our commitment to producing specialty chemicals that meet international industry standards, ensuring accurate composition and impurity-free chemicals. Our state-of-the-art manufacturing equipment and superior purity chemicals enable us to deliver fine quality products with a long shelf life.
With a focus on precision and customer satisfaction, Vinipul Chemicals Pvt. Ltd offers Zinc Chloride that is tailored to meet specific requirements. As prominent zinc chloride manufacturers in India, whether you need it for industrial applications or specialized uses, our Zinc Chloride consistently delivers the desired results. Additionally, our products are renowned for their stability and usability over extended periods, making them convenient for storage and reliable for long-term use.
By choosing Vinipul Chemicals Pvt. Ltd as your zinc chloride supplier, you can expect exceptional quality products. Our dedication to accuracy, purity, and reliability sets us apart in the industry. Experience the difference and contact us today to discuss your requirements and benefit from our unwavering commitment to excellence in specialty chemicals.
What is a Zinc Chloride Solution?
Zinc Chloride, with the chemical formula ZnCl2, is an inorganic compound that exists in various forms. It can form hydrates and appears as colourless or white crystalline solids, which are highly soluble in water. There are four forms of anhydrous zinc chloride and five known hydrates of this compound. It is worth noting that zinc chloride is hygroscopic and has the ability to deliquesce.
Zinc Chloride Solution has numerous applications, finding wide use in textile processing, metallurgical fluxes, and chemical synthesis. It plays a significant role in these industries due to its properties and versatility. It should be noted that apart from the rare mineral simonkolleite, which has the chemical composition Zn5(OH)8Cl2·H2O, there are no known minerals with the same composition as zinc chloride.
In summary, zinc chloride is an inorganic compound that can exist in different forms and is highly soluble in water. It has a variety of applications in industries such as textile processing, metallurgy, and chemical synthesis. While there is a rare mineral called simonkolleite with a similar composition, zinc chloride itself does not occur naturally as a mineral.
Zinc Chloride Details
This table provides information about Zinc Chloride, a chemical compound with the CAS No. 7646-85-7 and EC Number. 231-592-0. The table also lists various Zinc Chloride common name and synonyms. The table contains Zinc Chloride structure, Zinc Chloride solubility and molecular formula. Zinc Chloride pH. value is also mentioned in the table. Zinc Chloride is commonly used in various industries in different applications. It is also used in the pharmaceutical industry for various purposes.
Specifications
| Chemical name | Zinc Chloride |
| CAS No | 7646-85-7 |
| EC Number | 231-592-0 |
| Commercial name / Synonyms | Zinc chloride, Butter of zinc, Zinc(II) chloride, Zinc butter, Zinc chloride (ZnCl2), Zinkchloride, Zintrace |
| Molecular formula | ZnCl2 Cl2Zn |
| Chemical Structure | 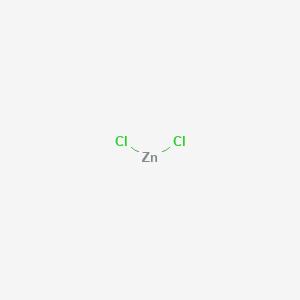 |
| Melting Point | 554 °F (NIOSH, 2022) |
| IUPAC Name | dichlorozinc |
| Component Compounds | NA |
| Physical Form | White, very deliquesce granules, or fused pieces or rods |
| Physical Appearance | Dry Powder; Dry Powder, Liquid; Liquid; Other Solid; Pellets or Large Crystals |
| Solubility | 435 % at 70 °F (NIOSH, 2022) |
| Solubility in water, g/100ml at 25 °C | 432 (very good) |
| pH | 1.0 for 6 M |
| Packaging Details | 25 kg / 50 kg / HDPE packaging bags / Drum/ As per Client’s requirements |
Computed Properties
The table provides various properties of a chemical compound, including its Zinc Sulphate molar mass, four hydrogen bond acceptor counts, an exact mass and mono-isotopic mass, heavy atom counts, and a topological polar surface area. These properties are useful in identifying and characterizing the compound for various purposes in industries such as pharmaceuticals and chemicals.
| Property Name | Property Value |
| Molecular Weight | 161.4 g/mol |
| Hydrogen Bond Acceptor Count | 4 |
| Exact Mass | 159.880871 g/mol |
| Monoisotopic Mass | 159.880871 g/mol |
| Heavy Atom Count | 6 |
| Topological Polar Surface Area | 88.6Ų |
| Complexity | 62.2 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
Related Compounds with Annotation
The table provides a summary of various structures and compounds related to zinc chloride. It includes compound names, unique identifiers such as Compound CID and UNII, as well as annotation types and neighbour types. The compounds listed range from zinc chloride with different isotopes to related variations and derivatives. The table also includes additional information such as alternative names, references to PubChem and other databases, and notations. Overall, the table presents a comprehensive overview of different forms and related compounds of zinc chloride.
Zinc Chloride Price
If you are looking to purchase Zinc Chloride, it’s important to know the current market price. Zinc Chloride price can vary depending on the various factors. Vinipul Chemicals offers the best price in the market listed below: –
| Product Range | Price |
| Zinc Chloride – Electroplating Grade | Rs 70/kg |
| Zinc Chloride 67% | Rs 80/kg |
| Zinc Chloride 35% | Rs 40/kg |
Prices shown above are provisional prices and may change due to different market conditions for latest prices
Call Us
+91-932 223 1817
Mail Us
business@vinipulchemicals.com
Types of Zinc Chloride Solution

Zinc Chloride - Electroplating Grade
Zinc chloride electroplating grade solution is specifically formulated for use in electroplating processes. Electroplating is a technique used to coat objects with a layer of metal, typically for decorative or protective purposes. The electroplating grade zinc chloride solution contains a high concentration of zinc chloride and other additives to ensure optimal plating results. It provides good conductivity and facilitates the adherence of the zinc coating onto the surface of the object being plated.

Zinc Chloride 67%
Zinc chloride 67% solution is a concentrated solution that contains 67% by weight of zinc chloride dissolved in water. It is a highly corrosive and hygroscopic liquid. This solution finds applications in various industrial processes, including as a catalyst, a flux for soldering, a dehydrating agent, and a disinfectant. It can also be used in the production of batteries, textiles, and dyes. Due to its high concentration, it should be handled with care, as it can cause skin and eye irritation.

Zinc Chloride 35%
Zinc chloride 35% solution contains 35% by weight of zinc chloride dissolved in water. This solution has a lower concentration compared to the 67% solution. It is commonly used in industries such as pharmaceuticals, chemical manufacturing, and laboratory settings. Zinc chloride 35% solution can be utilized as a Lewis acid catalyst, a dehydrating agent, and in various organic synthesis reactions. It can also be employed in the treatment of wood, as a preservative and fire retardant.
Zinc Chloride Solution Manufacturing Process
The manufacturing process of Zinc Chloride Solution involves several steps to ensure the production of high-quality and pure zinc chloride. Here is a general overview of the typical manufacturing process:
- Zinc Sourcing: The process begins with the sourcing of zinc, which can be obtained from various sources such as zinc ores, zinc scrap, or recycled materials. The selected zinc source undergoes thorough quality control to ensure its suitability for the production of zinc chloride.
- Purification: The sourced zinc is then subjected to purification processes to remove impurities and contaminants. This may involve processes such as roasting, smelting, or electrolysis to obtain pure zinc metal.
- Chlorination: The purified zinc is then reacted with chlorine gas to form zinc chloride. This reaction typically takes place in a controlled environment, such as a reactor or furnace, where the zinc and chlorine are brought together under specific temperature and pressure conditions.
- Cooling and Solidification: After the chlorination process, the resulting zinc chloride vapor is cooled and condensed to form solid zinc chloride crystals. This cooling process allows the vapor to transition into a solid state, resulting in the formation of fine quality zinc chloride crystals.
- Drying and Packaging: The obtained zinc chloride crystals are then subjected to drying processes to remove any remaining moisture. This is essential to ensure the long shelf life and stability of the product. Once dried, the zinc chloride is carefully packaged in suitable containers, such as bags or drums, to preserve its quality during storage and transportation.
Throughout the manufacturing process, quality control measures are implemented to monitor the composition, purity, and other specifications of the zinc chloride. This ensures that the final product meets the required industry standards and customer expectations.
It’s important to note that specific manufacturing techniques and variations may exist based on the desired grade and application of the zinc chloride being produced.
Is ZnCl2 a reducing agent?
In the compound ZnCl2, the zinc atom has an oxidation state of +2, and when it reacts with HCl, it loses electrons and undergoes oxidation. At the same time, the hydrogen in HCl is reduced, as its oxidation state decreases from +1 to 0. In this context, ZnCl2 acts as an oxidizing agent, facilitating the oxidation of zinc.
Is ZnCl2 a catalyst?
Yes, Zinc Chloride (ZnCl2) is commonly used as a catalyst in organic synthesis reactions. As a Lewis acid catalyst, it can facilitate various chemical reactions by accepting electron pairs from other molecules and promoting bond formation or rearrangement.
Industrial Applications
Zinc chloride solution finds extensive use in various industrial applications due to its versatile properties. Here are some common zinc chloride uses:

Textile Industry
In the textile industry, zinc chloride serves as a catalyst for the production of viscose rayon fibers. It aids in the conversion of cellulose into a soluble form, allowing for the creation of synthetic fibers used in textiles, clothing, and other fabric-based products.

Chemical Synthesis
Zinc chloride solution is a crucial reagent in various chemical synthesis reactions. It is commonly employed as a catalyst or activator in organic reactions, including Friedel-Crafts acylation and cyclization reactions. Additionally, it is used in the synthesis of dyes, pigments, and pharmaceutical compounds.

Battery Manufacturing
Zinc chloride is a key ingredient in the production of dry cell batteries. It acts as an electrolyte, facilitating the flow of ions between the battery electrodes, thus enabling the battery to generate electrical energy.

Metal Flux
As a fluxing agent, zinc chloride promotes the removal of oxides and impurities from metal surfaces during soldering, welding, and brazing processes. It improves the wetting characteristics of the molten solder or metal, ensuring strong and reliable metal joints.
These are just a few examples of the wide-ranging industrial applications of zinc chloride. Its versatility, reactivity, and unique properties make it a valuable compound in numerous industrial processes across different sectors.
Where to buy Zinc Chloride Solution?
For those looking to purchase high-purity Zinc Chloride Solution, Vinipul Chemicals Pvt. Ltd. is the go-to source. As renowned Indian zinc chloride manufacturers, supplier, and exporter, we specialize in producing chemicals with precise composition and superior purity, adhering to international industry standards. By contacting Vinipul Chemicals directly through our website or sales team, customers can obtain detailed information about their Zinc Chloride products, including pricing, packaging options, and delivery terms. With our commitment to excellence and customer satisfaction, Vinipul Chemicals Pvt. Ltd. is a trusted and reliable zinc chloride suppliers for acquiring chemicals for various industrial applications.
FAQ's
A: Yes, Zinc Chloride is corrosive and can cause skin and eye irritation. Proper safety precautions should be taken while handling it.
A: Yes, Zinc Chloride finds applications in the pharmaceutical industry for various purposes, such as in the synthesis of drugs and as a catalyst in chemical reactions.
A: You can purchase Zinc Chloride from chemical suppliers, distributors, or manufacturers specializing in specialty chemicals. Vinipul Chemicals Pvt. Ltd. is a reputable manufacturer, supplier, and exporter of high-purity Zinc Chloride.
A: Yes, Zinc Chloride is available in various grades and purities, ranging from technical grade to high-purity grades depending on the specific application requirements.
A: The shelf life of Zinc Chloride can vary depending on factors such as storage conditions and packaging, but typically it has a relatively long shelf life when stored properly.
A: Yes, Zinc Chloride can be shipped internationally. However, it is important to comply with relevant regulations and requirements for safe transport and handling.
A: Yes, Zinc Chloride may have alternative names such as Zinc dichloride, Dichlorozinc, or Zinc(II) chloride
A: Yes, Zinc Chloride has some disinfectant properties and can be used in certain applications for its antimicrobial effects
A: Zinc Chloride can be harmful to aquatic life and should be handled and disposed of properly to prevent environmental contamination.
A: Yes, Zinc Chloride is used in water treatment processes for various purposes, such as removing impurities or as a coagulant in wastewater treatment.
A: Zinc Chloride should be stored in a cool, dry place, away from moisture and incompatible substances. Proper ventilation and sealed containers are recommended.
A: Zinc Chloride may react with certain chemicals, so it is important to check compatibility before mixing it with other substances or materials.
Market Area
We supply and exports Zinc Chloride Solution in all parts of the world such as
Zinc Chloride Solution in Africa Countries
South Africa , Nigeria, Kenya, Ghana, Ethiopia, Tanzania, Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic (CAR), Chad, Comoros, Democratic Republic of the Congo, Cote d’Ivoire, Djibouti, Egypt, Equatorial Guinea, Eritrea, Gabon, Gambia, Guinea, Guinea-Bissau, Lesotho, Liberia, Libya, Madagascar, Malawi, Mali, Mauritania, Mauritius, Morocco, Mozambique, Namibia, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, Somalia, South Sudan, Sudan, Swaziland, Togo, Tunisia, Uganda, Zambia, Zimbabwe.
Zinc Chloride Solution in Gulf Countries
Oman, Qatar, Kuwait, Saudi Arabia, Dubai, Bahrain, Iran, United Arab Emirates
Zinc Chloride Solution in Asia Countries
Afghanistan, Armenia, Azerbaijan, Bahrain, Bangladesh, Bhutan, Brunei, Cambodia, China, Cyprus, Georgia, India, Indonesia, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Laos, Lebanon, Malaysia, Maldives, Mongolia, Myanmar (Burma), Nepal, North Korea, Oman, Pakistan, Palestine, Philippines, Qatar, Russia, Saudi Arabia, Singapore, South Korea, Sri Lanka, Syria, Taiwan, Tajikistan, Thailand, Timor-Leste, Turkey, Turkmenistan, United Arab Emirates (UAE), Uzbekistan, Vietnam, Yemen
We supply Zinc Chloride Solution in all parts of India.
Andhra Pradesh, Arunachal Pradesh, Assam, Bihar, Chhattisgarh, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu & Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Manipur, Meghalaya, Mizoram, Nagaland, Odisha, Punjab, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Uttarakhand, Uttar Pradesh and West Bengal.
Note: – Please be advised that the information contained in this document is intended for illustrative purposes only. Due to variations in product grade, applications, industries, or uses, the accuracy of the information provided cannot be guaranteed. © Copyright 2023 © Vinipul Chemicals All Rights Reserved (Terms of Use). Reproduction of any material from this site is strictly prohibited without permission. Vinipul Chemicals products are exclusively sold through the company’s website. For precise product specifications and requirements, as well as advice on which products are best suited for your specific application needs, please contact us at business@vinipulchemicals.com Use Terms | Privacy.

