Calcium Acetate Manufacturer and Supplier
CAS No.: 62-54-4 | EC Number.: 200-540-9 | Molecular formula.: C4H6O4.Ca, Ca(C2H3O2)2, C4H6CaO4
Calcium Acetate
Calcium Acetate Manufacturers, Calcium Acetate Suppliers, Calcium Acetate Formula, Calcium Acetate Price, Calcium Acetate SDS, Calcium Acetate Exporter in Mumbai, India.
Vinipul Chemicals Pvt. Ltd., a renowned manufacturer, supplier, and exporter based in India, specializes in producing high-purity Calcium Acetate (CAS No. 62-54-4) – a specialized chemical compound. We are highly regarded for our commitment to maintaining accurate composition and ensuring that our Calcium Acetate is free from impurities.
To achieve such high quality, we utilize superior purity chemicals and employ the latest manufacturing equipment in accordance with international industry standards. At Vinipul Chemicals, our primary focus is on providing Calcium Acetate of exceptional quality, ensuring that it meets the exact composition requirements and possesses a long shelf life.
Our Calcium Acetate is meticulously formulated to meet stringent standards and is trusted by numerous customers worldwide. With our dedication to quality control and precision manufacturing processes, we have earned a reputation as one of the prominent calcium acetate manufacturers in the chemical industry.
As a leading calcium acetate suppliers, manufacturer and exporter, we take pride in offering fine-quality Ca(C2H3O2)2 to our esteemed customers. Whether you require Calcium Acetate for research purposes, industrial applications, or any other specific needs, Vinipul Chemicals Pvt. Ltd. is your trusted partner.
What is Calcium Acetate?
Calcium Acetate is a calcium salt derived from acetic acid and is represented by the chemical formula Ca(C2H3O2)2. In its anhydrous form, Calcium Acetate is highly hygroscopic, meaning it readily absorbs moisture from the surroundings. When the water content is removed from Calcium Acetate, it transforms into an anhydrous state or a powdered form, known as Calcium Acetate Anhydrous.
Calcium Acetate is commonly referred to as calcium acetate, while its systematic name is calcium ethanoate. In the past, it was also known as acetate of lime. The anhydrous form of calcium acetate exhibits high hygroscopicity, making it prone to absorbing moisture from the surroundings. As a result, the monohydrate form (Ca(CH3COO)2•H2O) is more commonly encountered.
Calcium Acetate Details
This table provides information about Calcium Acetate, a chemical compound with the CAS No. 557-34-6 and EC Number 209-170-2. The table also lists various Calcium Acetate common name and synonyms. The table contains Calcium Acetate structure, Calcium Acetate solubility and molecular formula. It is a white granular powder of monoclinic crystals. Calcium Acetate pH. value is also mentioned in the table. Calcium Acetate is commonly used in various industries in different applications. It is also used in the pharmaceutical industry for various purposes.
Specifications
| Chemical name | Calcium Acetate |
| CAS No | 62-54-4 |
| EC Number | 200-540-9 |
| Commercial name / Synonyms | Calcium acetate, 62-54-4, Calcium diacetate, Acetic acid, calcium salt, Lime acetate, Lime pyrolignite, Acetate of lime, Gray Acetate of Lime, Brown Acetate of Lime, calcium ethanoate, calcium;diacetate, Calcium acetate, anhydrous, Calcium di(acetate) |
| Molecular formula | C4H6O4.Ca Ca(C2H3O2)2 C4H6CaO4 |
| Chemical Structure | 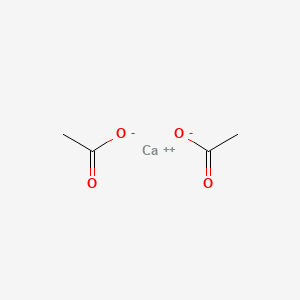 |
| Melting Point | > 160 °C |
| IUPAC Name | calcium-diacetate |
| Parent Compound | Acetic Acid |
| Component Compounds | Calcium Acetic Acid |
| Physical Form | Colourless Crystals |
| Physical Appearance | Dry Powder; Liquid; Other Solid; Pellets or Large Crystals |
| pH | 7.6 for 0.2M solution |
| Solubility | slightly soluble in methanol, hydrazine |
| Solubility in water | 37.4 g/100 mL (0 °C) 34.7 g/100 mL (20 °C) 29.7 g/100 mL (100 °C) |
| Packaging Details | 25 kg / 50 kg / HDPE packaging bags / Drum/ As per Client’s requirements |
Computed Properties
| Property Name | Property Value |
| Molecular Weight | 158.17 g/mol |
| Hydrogen Bond Acceptor Count | 4 |
| Exact Mass | 157.9891995 g/mol |
| Monoisotopic Mass | 157.9891995 g/mol |
| Heavy Atom Count | 9 |
| Topological Polar Surface Area | 80.3Ų |
| Complexity | 25.5 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
Related Compounds with Annotation
The table contains a list of compounds related to Calcium Acetate with their corresponding Compound CID, Neighbour Type, and Annotation Types Count. The compounds include Calcium acetate, Calcium acetate monohydrate, Ca(OAc)2, Calcium acetate hydrate, Renepho, OsvaRen, calcium acetate/magnesium carbonate, Nickel(cento) acetate, calcium acetate sodium, and calcium acetate potassium, among others. The Compound CID represents the unique identifier for each compound, the Neighbour Type indicates the two-dimensional structure, and the Annotation Types Count specifies the number of annotation types associated with each compound. The table provides a comprehensive overview of the compounds and their corresponding properties.
| Structure Image | Structure Name | Compound CID | Neighbour Type | Annotation Types Count |
|---|---|---|---|---|
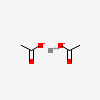 | Calcium acetate | 6116 | 2D | 12 |
 | Calcium acetate monohydrate | 82163 | 2D | 9 |
 | Ca(OAc)2 | 517040 | 2D | 5 |
 | Calcium acetate hydrate | 517438 | 2D | 2 |
 | Renepho, OsvaRen, calcium acetate/magnesium carbonate | 11514263 | 2D | 4 |
 | SCHEMBL8853412 | 19027274 | 2D | 1 |
 | NA | 19744921 | 2D | 1 |
 | NA | 21398261 | 2D | 1 |
 | NA | 22290020 | 2D | 1 |
 | Nickel(cento) acetate | 23207293 | 2D | 1 |
 | NA | 86654289 | 2D | 1 |
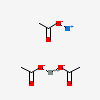 | calcium acetate sodium | 129637660 | 2D | 2 |
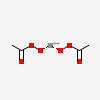 | NA | 129644897 | 2D | 1 |
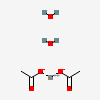 | NA | 129714401 | 2D | 2 |
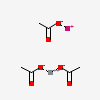 | calcium acetate potassium | 129819092 | 2D | 1 |
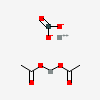 | NA | 140437785 | 2D | 1 |
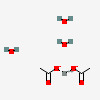 | NA | 140617651 | 2D | 1 |
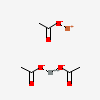 | NA | 155578873 | 2D | 1 |
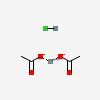 | NA | 155665772 | 2D | 1 |
 | NA | 157113883 | 2D | 1 |
 | NA | 157645415 | 2D | 1 |
 | NA | 157904544 | 2D | 1 |
 | NA | 158569878 | 2D | 1 |
 | NA | 158645458 | 2D | 1 |
 | NA | 158778168 | 2D | 1 |
 | NA | 159269223 | 2D | 1 |
 | NA | 159340621 | 2D | 1 |
 | NA | 159345671 | 2D | 1 |
 | NA | 159611285 | 2D | 1 |
 | NA | 159792690 | 2D | 1 |
 | NA | 160438633 | 2D | 1 |
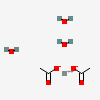 | NA | 160553766 | 2D | 1 |
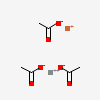 | NA | 161083599 | 2D | 1 |
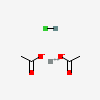 | NA | 161392559 | 2D | 1 |
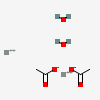 | NA | 161949439 | 2D | 1 |
 | NA | 162116296 | 2D | 1 |
 | NA | 163843950 | 2D | 1 |
 | NA | 164146532 | 2D | 1 |
Calcium Acetate Price
If you are looking to purchase Calcium Acetate, it’s important to know the current market price. Calcium Acetate price can vary depending on the various factors. Vinipul Chemicals offers the best price in the market listed below: –
| Product Range | Price |
| Calcium Acetate Anhydrous | Rs 120/kilogram |
| Calcium Acetate Monohydrate | Rs 120/kilogram |
Prices shown above are provisional prices and may change due to different market conditions for latest prices
Call Us
+91-932 223 1817
Mail Us
business@vinipulchemicals.com
Types of Calcium Acetate

Calcium Acetate Anhydrous
Calcium Acetate Anhydrous refers to the anhydrous or dry form of Calcium Acetate. It has a strong affinity for moisture and readily absorbs water from the surrounding environment. This characteristic makes it highly hygroscopic, which means it can easily attract and retain moisture. Due to its moisture-absorbing properties, Calcium Acetate Anhydrous is often used in applications where a dry or anhydrous state is required.

Calcium Acetate Monohydrate
On the other hand, Calcium Acetate Monohydrate is the form of Calcium Acetate that contains one molecule of water per calcium acetate unit. This form is commonly encountered and is more stable compared to the anhydrous form. The presence of water in Calcium Acetate Monohydrate affects its physical properties and behaviour, including solubility and stability. It is typically the more commercially.
Prices shown above are provisional prices and may change due to different market conditions for latest prices
Call Us
+91-932 223 1817
Mail Us
business@vinipulchemicals.com
How to Make Calcium Acetate?
The manufacturing process of Calcium Acetate is a multi-step procedure that ensures the production of high-quality and pure calcium acetate raw materials. The process begins by combining 1 part of the shell, which serves as a source of calcium, with 1-2 parts of glacial acetic acid and 3-5 parts of water. These components are carefully measured and mixed together.
Once the mixture is prepared, it is left to react for a minimum of 48 hours. During this period, the reaction occurs at normal temperature and pressure, while continuous agitation is maintained. This allows for optimal interaction between the shell, glacial acetic acid, and water, promoting the formation of calcium acetate.
After the reaction period, the mixture undergoes a series of important processing steps. Firstly, primary filtration is carried out to separate any solid impurities or undissolved particles from the liquid mixture. This step ensures the removal of unwanted substances, leaving behind a cleaner solution.
The next step is fine filtration, which further refines the liquid by removing any remaining impurities or suspended solids. This process helps to achieve a higher level of purity in the calcium acetate solution.
To enhance the appearance and quality of the final product, a discoloration step is performed. This step involves the use of specific techniques or agents to eliminate any coloration or discoloration present in the solution, resulting in a clear and colourless liquid.
Finally, the liquid solution is subjected to a drying process to remove the water content. This is crucial because the anhydrous form of Calcium Acetate, which is highly hygroscopic, is desired for certain applications. By eliminating water, the Calcium Acetate is transformed into an anhydrous state or a powdered form known as Calcium Acetate Anhydrous.
Is Calcium Acetate Soluble in Water?
Yes, Calcium Acetate is soluble in water. When Calcium Acetate comes into contact with water, it dissolves and forms an aqueous solution. This solubility in water is one of the reasons why Calcium Acetate is commonly used in various applications, such as food additives, pharmaceuticals, and water treatment processes.
Is calcium acetate a vitamin?
Calcium Acetate serves as a calcium supplement specifically designed to regulate and control the level of phosphate in the blood. It is commonly prescribed for patients undergoing dialysis treatment as a result of severe kidney disease. When the kidneys are not functioning properly, they struggle to effectively filter and remove excess phosphate from the bloodstream. Elevated levels of phosphate in the blood, known as hyperphosphatemia, can lead to complications and adverse effects on the body, particularly in individuals with kidney disease.
Calcium Acetate works by binding to the excess phosphate in the gastrointestinal tract, preventing its absorption into the bloodstream. This mechanism helps to reduce the phosphate levels and maintain them within a healthy range. By effectively managing phosphate levels, Calcium Acetate helps prevent complications associated with hyperphosphatemia, such as bone and mineral disorders.
Patients on dialysis often require calcium supplementation due to the removal of calcium during the dialysis process. Calcium Acetate serves as an essential source of calcium, replenishing the body’s calcium stores and ensuring the proper functioning of vital physiological processes.
Is calcium acetate a preservative?
Yes, Calcium Acetate is classified as a preservative according to the Codex Alimentarius, which is an international food standards organization. It is listed as a food additive in the Codex General Standard for Food Additives (GSFA) and is assigned functional class designations that include acidity regulator, preservative, and stabilizer.
As a preservative, Calcium Acetate helps extend the shelf life of certain food products by inhibiting the growth of spoilage-causing microorganisms. It works by creating an environment that is unfavorable for the growth and proliferation of bacteria, molds, and yeasts, thereby preserving the quality and freshness of the food.
It is important to note that while Calcium Acetate can function as a preservative, its primary role in food applications may vary depending on the specific product and intended use. Different food regulations in various countries may also provide specific guidelines and limitations for the use of Calcium Acetate as a preservative in different food products.
What happens when calcium acetate is heated?
When calcium acetate (CH3COO)2Ca is heated, it undergoes decomposition, resulting in the formation of acetone (CH3COCH3) and calcium carbonate (CaCO3). This reaction is commonly employed in the industrial production of acetone.
During the heating process, calcium acetate breaks down into acetone and calcium carbonate through a chemical reaction known as thermal decomposition. The reaction is typically conducted under controlled conditions with slow heating, and it does not involve any specific codification.
The production of acetone from calcium acetate through heating is a valuable industrial process. Acetone is a widely used organic solvent with various applications in industries such as pharmaceuticals, cosmetics, plastics, and chemical manufacturing.
It is important to note that the thermal decomposition of calcium acetate is a specific reaction and should be performed with proper equipment and expertise to ensure safety and efficient production.
What is the difference between calcium carbonate and calcium acetate?
The key difference between calcium acetate and calcium carbonate is that calcium acetate contains a lower amount of elemental calcium, whereas calcium carbonate contains a high amount of elemental calcium.
Industrial Applications
Calcium Acetate finds applications in various industries due to its properties and chemical characteristics. Some of the common industrial applications of Calcium Acetate include:

Food and Beverage Industry
Calcium Acetate is used as a food additive, particularly as a preservative and acidity regulator. It helps extend the shelf life of certain food products and maintains their freshness. It is also utilized in the production of confectioneries, bakery goods, and dairy products.

Pharmaceutical Industry
Calcium Acetate is used in pharmaceutical formulations, primarily as a medication to treat hyperphosphatemia in patients with kidney disease. It helps in reducing excessive phosphate levels in the blood. Calcium Acetate is also employed in the production of calcium supplements and as a pharmaceutical excipient in tablet formulations.

Water Treatment
Calcium Acetate is utilized in water treatment processes as a coagulating agent to remove impurities and suspended particles. It aids in the clarification and purification of water, especially in municipal water treatment plants and industrial wastewater treatment facilities.

Textile Industry
Calcium Acetate is employed as a mordant in the dyeing and printing of textiles. It helps to fix and enhance the color fastness of dyes on fabrics, ensuring long-lasting and vibrant colours.

Construction Industry
Calcium Acetate is utilized in the construction sector as a concrete accelerator. It promotes the setting and hardening of cement, allowing for faster curing times and improved strength development.

Chemical Manufacturing
Calcium Acetate serves as a precursor or intermediate in the synthesis of various chemicals. It is used in the production of acetone, acetic acid, acetic anhydride, and other organic compounds.

Laboratory and Research
Calcium Acetate is commonly employed in laboratory settings for various purposes such as analytical chemistry, chemical reactions, and as a reagent in scientific experiments.
These are just a few examples of the industrial applications of Calcium Acetate. Its versatility and usefulness in different industries make it a valuable compound with a wide range of practical uses.
What is calcium acetate used for?
Calcium Acetate finds applications in various industries for its versatile properties. Some of the key calcium acetate uses include:
- Food Production: Calcium Acetate is used in a wide range of food products, including candy, confections, cake batters, pie fillings, puddings, certain cheeses, gelatine, snack foods, sweet sauces, bread, and baked goods. Food manufacturers sometimes add calcium acetate as a texturizer, thickening agent, and firming agent. It also acts as a growth inhibitor for certain bacteria, extending the shelf life of food products.
- Beverage Manufacturing: Calcium Acetate is employed in the production of beverages as an additive or ingredient.
- Health and Personal Care: Calcium Acetate is utilized in the health and personal care industry, particularly as a fragrance ingredient.
- pH Control in Food Processing: Calcium Acetate acts as a buffer in controlling the pH of food during processing, serving as an acidity regulator.
- Agriculture/Animal Feed/Poultry: Calcium Acetate Monohydrate can be used as a calcium supplement in pet foods and in agriculture as an animal feed additive. It helps provide necessary calcium to support animal health.
- Stabilizer in Packaging: Calcium Acetate functions as a stabilizer when salts migrate from food-packaging materials, acting as a sequestrant.
These diverse industrial applications demonstrate the versatility and usefulness of Calcium Acetate in different sectors. It contributes to the quality, stability, and functionality of various products across industries.
Where to buy calcium acetate?
If you are looking to buy calcium acetate then Vinipul Chemicals Pvt. Ltd. is a reputable calcium acetate manufacturer, supplier, and exporter based in Mumbai, India. We are well-known for our expertise in producing high-purity Calcium Acetate, a specialized chemical compound. Our commitment to delivering exceptional quality has earned us a prominent position in the industry.
With a focus on international markets, we export our premium Calcium Acetate to various regions, including across India and the Indian Subcontinent, East Asia, South East Asia, and the Middle East. Our products meet stringent quality standards, ensuring accurate composition, and are manufactured using state-of-the-art equipment and superior purity chemicals.
As a customer-centric organization, we take pride in offering reliable and consistent supplies of Calcium Acetate, catering to the diverse needs of our clients. With our strong presence and dedication to quality, Vinipul Chemicals Pvt. Ltd. has become a trusted name in the chemical industry, serving as a reliable partner for businesses worldwide.
FAQ's
The pH of this solution is between 4.5 and 5.5.
The solid-liquid ratio of 1:12 is selected as the best condition for preparing calcium acetate.
Calcium acetate is not listed for organic production.
Calcium acetate is generally safe for consumption when used as directed by a healthcare professional. However, it’s important to follow the recommended dosage and guidelines provided by your doctor to avoid any potential risks or complications.
Calcium acetate works by binding with dietary phosphate in the gastrointestinal tract, forming an insoluble compound that cannot be absorbed by the body. This helps to reduce the amount of phosphate that is absorbed into the bloodstream, thereby controlling phosphate levels.
The recommended dosage and administration guidelines for calcium acetate can vary depending on the individual’s specific condition and the healthcare professional’s instructions.
Some potential side effects of calcium acetate may include constipation, upset stomach, nausea, vomiting, and loss of appetite. Additionally, calcium acetate may interact with certain medications, so it’s important to inform your healthcare provider about all the medications you are taking to avoid any potential interactions.
High-quality calcium acetate can be purchased from various sources, including pharmacies, pharmaceutical suppliers, chemical supply companies such as Vinipul Chemicals Pvt. Ltd. It’s important to ensure that you are obtaining it from a reputable source to ensure its quality and safety.
The shelf life of calcium acetate can vary depending on the specific manufacturer and storage conditions. Generally, it has a relatively long shelf life of several years when stored properly in a cool, dry place and in its original packaging.
Calcium acetate should be stored in a tightly closed container, protected from moisture and humidity.
Yes, calcium acetate can be used in some pharmaceutical formulations for purposes other than phosphate control. However, its usage in such formulations would depend on specific formulation requirements and the judgment of the healthcare professional or pharmacist overseeing the treatment.
Market Area
We supply and exports Calcium Acetate in all parts of the world such as
Calcium Acetate in Africa Countries
South Africa , Nigeria, Kenya, Ghana, Ethiopia, Tanzania, Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic (CAR), Chad, Comoros, Democratic Republic of the Congo, Côte d’Ivoire, Djibouti, Egypt, Equatorial Guinea, Eritrea, Gabon, Gambia, Guinea, Guinea-Bissau, Lesotho, Liberia, Libya, Madagascar, Malawi, Mali, Mauritania, Mauritius, Morocco, Mozambique, Namibia, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, Somalia, South Sudan, Sudan, Swaziland, Togo, Tunisia, Uganda, Zambia, Zimbabwe.
Calcium Acetate in Gulf Countries
Oman, Qatar, Kuwait, Saudi Arabia, Dubai, Bahrain, Iran, United Arab Emirates
Calcium Acetate in Asia Countries
Afghanistan, Armenia, Azerbaijan, Bahrain, Bangladesh, Bhutan, Brunei, Cambodia, China, Cyprus, Georgia, India, Indonesia, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Laos, Lebanon, Malaysia, Maldives, Mongolia, Myanmar (Burma), Nepal, North Korea, Oman, Pakistan, Palestine, Philippines, Qatar, Russia, Saudi Arabia, Singapore, South Korea, Sri Lanka, Syria, Taiwan, Tajikistan, Thailand, Timor-Leste, Turkey, Turkmenistan, United Arab Emirates (UAE), Uzbekistan, Vietnam, Yemen
We supply Calcium Acetate in all parts of India.
Andhra Pradesh, Arunachal Pradesh, Assam, Bihar, Chhattisgarh, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu & Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Manipur, Meghalaya, Mizoram, Nagaland, Odisha, Punjab, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Uttarakhand, Uttar Pradesh and West Bengal.
Note: – Please be advised that the information contained in this document is intended for illustrative purposes only. Due to variations in product grade, applications, industries, or uses, the accuracy of the information provided cannot be guaranteed. © Copyright 2023 © Vinipul Chemicals All Rights Reserved (Terms of Use). Reproduction of any material from this site is strictly prohibited without permission. Vinipul Chemicals products are exclusively sold through the company’s website. For precise product specifications and requirements, as well as advice on which products are best suited for your specific application needs, please contact us at business@vinipulchemicals.com Use Terms | Privacy.


