Sodium Acetate Manufacturer and Supplier
CAS No.: 127-09-3 | EC Number.: 204-823-8 | Molecular formula.: C2H3NaO2
Sodium Acetate
Sodium Acetate Manufacturers, Sodium Acetate Suppliers, Sodium Acetate Formula, Sodium Acetate Price, Sodium Acetate Exporter in Mumbai, India.
Vinipul Chemicals Pvt. Ltd. is a renowned manufacturer, supplier, and exporter of high-purity Sodium Acetate (CAS No. 127-09-3), specializing in the production of specialty chemicals. Our company is based in India and is widely recognized for providing C2H3NaO2 with exceptional quality and accuracy in composition.
At Vinipul Chemicals, we prioritize the use of superior purity chemicals and the latest manufacturing equipment to ensure the production of Sodium Acetate offered by sodium acetate manufacturers meets international industry standards. Our commitment to maintaining high standards of purity guarantees that our Sodium Acetate is free from impurities, making it ideal for various applications.
We take pride in offering Sodium Acetate with precise compositions tailored to meet specific requirements. Whether you need Sodium Acetate for research purposes, industrial use, or any other application, we can provide you with the desired composition and concentration.
With Vinipul Chemicals Pvt. Ltd as your sodium acetate manufacturer, you can have confidence in the quality and reliability of our Sodium Acetate. Choose Vinipul Chemicals Pvt. Ltd a leading sodium acetate supplier for high-purity products that fulfils your specific requirements, adheres to international standards, and offers excellent quality, precise composition, and a long shelf life.
What is Sodium Acetate?
Sodium acetate (CH3COONa) is the sodium salt of acetic acid, which is produced as a by-product during the manufacturing of acetanilide and acetylsalicylic acid. The sodium acetate formula is commonly prepared by neutralizing acetic acid with sodium carbonate and then evaporating the solution to form crystallized sodium acetate. This compound exists as a white granular powder composed of monoclinic crystals and is a colourless, deliquescent salt. Sodium acetate chemical formula has a wide range of applications, particularly in the production of sodium bicarbonate and sodium hydroxide. In the industry, it is typically derived from glacial acetic acid and sodium hydroxide.
Sodium Acetate Details
This table provides information about Sodium Acetate, a chemical compound with the CAS No. 127-09-3 and EC Number 204-823-8. The table also lists various Sodium Acetate common name and synonyms. The table contains sodium acetate structure and molecular formula. It is a white granular powder of monoclinic crystals. Sodium Acetate pH. value is also mentioned in the table. Sodium Acetate is commonly used in various industries in different applications. It is also used in the pharmaceutical industry for various purposes.
Specifications
| Chemical name | Sodium Acetate |
| CAS No | 127-09-3 |
| EC Number | 204-823-8 |
| Commercial name / Synonyms | Acetic acid, Sodium acetate anhydrous, sodium salt, Acetic acid sodium salt, Sodium acetate anhydrous, Anhydrous sodium acetate, Sodium ethanoate, sodium-acetate NaOAc, Acetic acid, sodium salt (1:1), SODIUM DIACETATE |
| Molecular formula | C2H3NaO2 |
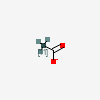
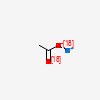
| Chemical Structure |  |
| Melting Point | 324.0 °C |
| IUPAC Name | sodium-acetate |
| Parent Compound | Acetic Acid |
| Component Compounds | Sodium Acetic Acid |
| Physical Form | White Granular Powder of Monoclinic Crystals |
| Physical Appearance | Dry Powder / Liquid / Pellets / Large Crystals / Wet Solid |
| pH of 5% solution | 7.5 – 9.0 |
| Odour | Vinegar (acetic acid) odour when heated to decomposition |
| Crystal structure | Monoclinic |
| Solubility in alcohol | Soluble in alcohol, hydrazine, SO2 |
Solubility 5% solution in water) | Clear solution |
| Packaging Details | 25 kg / 50 kg / HDPE packaging bags / Drum/ As per Client’s requirements |
Computed Properties
| Property Name | Property Value |
| Molecular Weight | 82.03 g/mol |
| Hydrogen Bond Acceptor Count | 2 |
| Exact Mass | 82.00307362 |
| Monoisotopic Mass | 82.00307362 |
| Heavy Atom Count | 5 |
| Topological Polar Surface Area | 40.1 Ų |
| Complexity | 34.6 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | `Yes | `
Related Compounds with Annotation
The table consists of related compounds with annotations. The compounds listed include various forms of acetate and its derivatives. Some notable compounds include Acetate, Sodium Acetate, Sodium hydrogen di(2H3) acetate, Acetoxy radical, Sodium peracetate, 2-Deuterioacetate, and Thorium acetate, among others. The molecular weight, molecular formula, and Compound CID (Chemical Identifier) are provided for each compound. The table also includes isotopically labelled compounds and different salts of acetate with various cations such as sodium, potassium, aluminium, and thorium. These compounds offer a range of chemical variations and isotopic labelling, which can be useful for various research and analytical purposes.
| Structure | Compound CID | Name | Molecular Weight, g/mol | Molecular Formula |
|---|---|---|---|---|
 | 175 | Acetate | 59.04 | C2H3O2- |
 | 517045 | Sodium Acetate | 82.03 | C2H3NaO2 |
 | 174016 | CID 174016 | 98.03 | C2H3NaO3 |
 | 450346 | CID 450346 | 58.045 | C2H3O2- |
 | 450348 | CID 450348 | 58.045 | C2H3O2- |
 | 16211391 | Sodium hydrogen di(2H3)acetate | 85.05 | C2H3NaO2 |
 | 5359261 | Acetoxy radical | 59.04 | C2H3O2 |
 | 12358161 | CID 12358161 | 63.029 | C2H3O2- |
 | 16211957 | Natriumacetat-2-14C | 84.026 | C2H3NaO2 |
 | 16211894 | Sodium acetate-2-(13-C),d3 | 86.045 | C2H3NaO2 |
 | 16211895 | Sodium acetate-13C2,d3 | 87.038 | C2H3NaO2 |
 | 16211956 | Acetic-1-(14)C acid, sodium salt | 84.026 | C2H3NaO2 |
 | 23674263 | Sodium peracetate | 98.03 | C2H3NaO3 |
 | 18350099 | Potassium;sodium;acetate | 121.13 | C2H3KNaO2+ |
 | 18609027 | 2-Deuterioacetate | 60.05 | C2H3O2- |
 | 18987058 | Trisodium;acetate | 128.009 | C2H3Na3O2+2 |
 | 21909732 | Disodium;acetate | 105.02 | C2H3Na2O2+ |
 | 23663393 | Acetic-t acid, sodium salt | 84.04 | C2H3NaO2 |
 | 23715518 | Acetic acid, aluminium sodium salt | 109.02 | C2H3AlNaO2+3 |
 | 23681129 | Sodium acetate-1-13C | 83.026 | C2H3NaO2 |
 | 23681130 | Sodium (2-13C) acetate | 83.026 | C2H3NaO2 |
 | 23681132 | Sodium acetate-13C2 | 84.019 | C2H3NaO2 |
 | 57817008 | 2,2,2-Tritritioacetate | 65.069 | C2H3O2- |
 | 23717929 | Sodium acetate C-11 | 81.035 | C2H3NaO2 |
 | 23717931 | Acetic acid,sodium salt,[1,2-14c] | 86.019 | C2H3NaO2 |
 | 57641842 | Sodium;carbanide;acetate | 97.07 | C3H6NaO2- |
 | 71311149 | Sodium;acetate | 86.03 | C2H3NaO2 |
 | 74087785 | Sodium;methane;acetate | 98.08 | C3H7NaO2 |
 | 74765071 | Thorium;acetate | 291.08 | C2H3O2Th- |
 | 86584106 | Sodium monochloro acetate | 117.49 | C2H3ClNaO2 |
 | 86611583 | Sodium iodo acetate | 208.94 | C2H3INaO2 |
 | 87329169 | CID 87329169 | 105.02 | C2H3Na2O2 |
 | 101359700 | 2,2,2-Trideuterioacetate | 62.06 | C2H3O2- |
 | 102089142 | CID 102089142 | 61.029 | C2H3O2- |
 | 102601172 | Sodium deutero-acetate | 83.04 | C2H3NaO2 |
 | 131728846 | Sodium oxide acetate | 98.03 | C2H3NaO3-2 |
 | 146028092 | sodium;(2S)-2-deuterio-2-tritioacetate | 85.05 | C2H3NaO2 |
 | 153693916 | CID 153693916 | 61.037 | C2H3O2- |
 | 153693974 | CID 153693974 | 60.037 | C2H3O2- |
 | 153807963 | CID 153807963 | 66.068 | C2H3O2- |
 | 154058596 | CID 154058596 | 60.037 | C2H3O2- |
 | 154064598 | CID 154064598 | 61.037 | C2H3O2- |
 | 154410204 | 2,2-Dideuterioacetate | 61.06 | C2H3O2- |
 | 157942307 | Sodium;hydride;acetate | 83.04 | C2H4NaO2- |
 | 158339250 | CID 158339250 | 92.85 | C2H3BNaO2 |
 | 160080304 | Calcium;sodium;oxygen(2-);acetate | 138.11 | C2H3CaNaO3 |
 | 160114372 | Sodium;hydride;acetate | 85.06 | C2H6NaO2-3 |
 | 161338078 | CID 161338078 | 84.03 | C2H3NaO2 |
 | 163893100 | Magnesium;sodium;oxygen(2-);acetate | 122.34 | C2H3MgNaO3 |
Sodium Acetate Price
If you are looking to purchase Sodium Acetate, it’s important to know the current market sodium acetate price per gram. Sodium Acetate price can vary depending on the various factors. Vinipul Chemicals offers the best price in the market listed below: –
| Product Range | Price |
| Sodium Acetate Trihydrate | Rs 32/kilogram |
| Sodium Acetate Anhydrous | Rs 50/kilogram |
| Sodium Acetate Crystal | Rs 55/kilogram |
| Sodium Acetate Powder | Rs 20/kilogram |
| Sodium Acetate 99% | Rs 33/ kilogram |
| Sodium Acetate Anhydrous 99% | Rs 50/kilogram |
| Sodium Acetate Trihydrate 58-60% | Rs 32/ kilogram |
Prices shown above are provisional prices and may change due to different market conditions for latest prices
Call Us
+91-932 223 1817
Mail Us
business@vinipulchemicals.com
Types of Sodium Acetate

Sodium Acetate Trihydrate
This is a form of sodium acetate that contains three molecules of water for each molecule of sodium acetate. It appears as colourless crystals and has a molecular formula of C2H9NaO5. It is commonly used in various applications such as buffers, pH control, and as a food additive.

Sodium Acetate Anhydrous
Sodium acetate anhydrous is the anhydrous (without water) form of sodium acetate. It does not contain any water molecules and has the molecular formula C2H3NaO2. It is a white crystalline powder and is widely used in industries such as pharmaceuticals, dyes, and as a food preservative.

Sodium Acetate Crystal
Sodium acetate crystal refers to sodium acetate in its crystalline form. It can be either the Trihydrate or anhydrous form and is characterized by its crystal structure. Sodium acetate crystals are often used in heat packs or hand warmers, as they have the ability to undergo a rapid exothermic crystallization.

Sodium Acetate Powder
Sodium acetate powder refers to the finely ground form of sodium acetate. It can also be either the Trihydrate or anhydrous form. Sodium acetate powder is commonly used in various applications such as analytical chemistry, laboratory work, and as an ingredient in certain food products.

Sodium Acetate 99%
These terms indicate that the sodium acetate being referred to has a purity level of 99%. It means that 99% of the compound is composed of sodium acetate, while the remaining 1% may consist of impurities or water content in the case of the Trihydrate form.

Sodium Acetate Anhydrous 99%
These terms indicate that the sodium acetate being referred to has a purity level of 99%. It means that 99% of the compound is composed of sodium acetate, while the remaining 1% may consist of impurities or water content in the case of the Trihydrate form.

Sodium Acetate Trihydrate 58-60%
This term specifies the water content in the sodium acetate Trihydrate. It indicates that the Trihydrate contains 58-60% water by weight, while the remaining portion is sodium acetate. This information is relevant for applications where precise water content is necessary, such as in the formulation of specific solutions or reagents.
How to Make Sodium Acetate?
Sodium acetate manufacturing process includes:
- Start with vinegar: Sodium Acetate can be synthesized from common household vinegar, which is a diluted acetic acid solution.
- Choose your sodium source: You will need a sodium compound to react with the acetic acid. Sodium bicarbonate (baking soda) or sodium hydroxide are commonly used.
- Mixing the ingredients: In a container, combine the vinegar (acetic acid) with the sodium compound. Stir the mixture gently to ensure thorough mixing.
- Reaction and heating: The reaction between the acetic acid and sodium compound will release carbon dioxide gas and form Sodium Acetate. Apply gentle heat to the mixture, either by using a hot plate or heating it on a stovetop.
- Evaporation: Continue heating the mixture until most of the liquid has evaporated. This will help concentrate the Sodium Acetate solution.
- Crystallization: Allow the remaining liquid to cool down slowly. As it cools, Sodium Acetate crystals will start to form. You can aid the crystallization process by scratching the inside of the container with a glass rod or adding a small Sodium Acetate crystal as a seed.
- Harvesting the crystals: Once the crystallization process is complete, carefully collect the Sodium Acetate crystals using a filter or strainer. Rinse the crystals with a small amount of cold distilled water to remove any impurities.
- Drying the crystals: Place the harvested Sodium Acetate crystals on a paper towel or a clean, dry surface to air-dry. Avoid exposure to moisture during the drying process.
- Storage: Once the crystals are completely dry, transfer them to an airtight container to prevent moisture absorption. Store Sodium Acetate in a cool and dry place.
Note: It’s important to follow appropriate safety measures when handling chemicals and heating substances. Wear protective gloves, goggles, and work in a well-ventilated area to ensure your safety.
Is CH3COONa an acid or base?
The question that often arises is sodium acetate acid or base? Sodium Acetate is formed through a neutralization reaction between acetic acid (CH3COOH) and sodium hydroxide (NaOH). Acetic acid is a weak acid, meaning it only partially dissociates in water and does not completely release all of its hydrogen ions (H+). Sodium hydroxide, on the other hand, is a strong base that readily dissociates in water, releasing hydroxide ions (OH-).
During the neutralization reaction, the sodium hydroxide donates its hydroxide ions to the acetic acid. The hydrogen ion from the acetic acid combines with the hydroxide ion from the sodium hydroxide to form water (H2O). The remaining components, sodium (Na+) and the acetate ion (CH3COO-), combine to form the salt Sodium Acetate (CH3COONa).
The acetate ion (CH3COO-) derived from the weak acid acetic acid is a conjugate base. It can accept a proton (H+) from water, making it capable of acting as a weak base. When Sodium Acetate is dissolved in water, it undergoes ionization, releasing the acetate ions into the solution. These acetate ions can react with water molecules, accepting a proton and forming hydroxide ions (OH-). This process contributes to the basic properties of Sodium Acetate.
Therefore, due to the presence of the acetate ion, which can accept protons and generate hydroxide ions in solution, CH3COONa is considered a basic salt. It exhibits weak basic behaviour when dissolved in water.
Is sodium acetate a salt or acid?
Sodium acetate is a type of compound that falls under the category of salts. It is an organic sodium salt specifically, which means it contains sodium ions (Na+) and acetate ions (CH3COO-). Sodium acetate is commonly represented by its chemical formula, CH3COONa. In terms of its properties, sodium acetate is a hygroscopic powder, meaning it has a tendency to absorb moisture from its surroundings. It is highly soluble in water, which allows it to readily dissolve and dissociate into sodium and acetate ions when mixed with water.
Sodium acetate is widely used in various applications. One of its common uses is as a food additive, where it acts as a preservative, flavouring agent, or pH regulator. It is also utilized in the production of pharmaceuticals, dyes, and as a buffering agent in chemical laboratories. Furthermore, sodium acetate can be found in heating pads or hand warmers, where it undergoes a process called “heat of crystallization” to release heat when activated. Overall, sodium acetate is considered a salt due to its composition of sodium and acetate ions.
Is sodium acetate food safe?
Sodium acetate is generally recognized as safe (GRAS) for use in food according to the U.S. Food and Drug Administration (FDA). It is considered safe for consumption and its use is not restricted in most countries. Sodium acetate is commonly used in the food industry as a food additive, flavouring agent, preservative, and pH regulator.
One of the reasons sodium acetate is used in food is its ability to prevent the growth of bacteria in acidic conditions. It acts as a preservative by inhibiting the growth of microorganisms, helping to extend the shelf life of certain food products.
Sodium acetate also functions as a buffer, which means it helps maintain a stable pH level in food. It can prevent large fluctuations in acidity or alkalinity, ensuring that the food maintains its desired flavour and stability.
While acetic acid is often chosen when a sour taste is desired in food, the combination of sodium acetate and acetic acid can create a milder taste. This combination can provide a more balanced and less intense sour flavour profile, which may be preferred in certain food applications.
It’s worth noting that as with any food ingredient, individual sensitivities or allergies can vary. If you have specific concerns or dietary restrictions, it is recommended to check food labels and consult with healthcare professionals or nutritionists for personalized advice.
Is sodium acetate a preservative?
Sodium acetate is a substance that serves as a preservative in various food products, including preserved nuts and legumes. It is also a common ingredient found in peanut butter. Additionally, sodium acetate is used as a preservative in canned meat and deli meats. When it comes to canned fish, such as sardines, fish pastes, and fermented shellfish, sodium acetate is utilized to preserve the meat in these products.
Preservatives like sodium acetate are employed in food manufacturing to inhibit the growth of bacteria, fungi, and other microorganisms. By doing so, they help extend the shelf life of the products and maintain their quality and safety for consumption. Sodium acetate, in particular, is effective in preventing spoilage and maintaining the texture and flavour of preserved nuts, legumes, peanut butter, canned meat, deli meats, canned fish, and other related food items.
It’s important to note that the use of sodium acetate as a food preservative is regulated and approved by food safety authorities to ensure its safety for consumption within specified limits.
Is sodium acetate soluble in water?
Yes, Sodium acetate is a compound that exhibits high solubility in water. When sodium acetate is added to water, it readily dissolves, meaning it disperses evenly throughout the water molecules. The resulting solution is clear and colourless, indicating the complete dissolution of sodium acetate particles.
Is sodium acetate a strong base?
Sodium acetate is a salt resulting from the combination of a weak acid, acetic acid, and a strong base, sodium hydroxide (NaOH).
Is sodium acetate good for cleaning?
Sodium acetate, a highly effective cleaning agent, swiftly eliminates unsightly impurities like stains, rust, and scale from metal surfaces. With its remarkable stain-removing capabilities, it effortlessly breaks down and lifts even the stubbornest discolorations, revealing the metal’s pristine beauty. By converting rust into a soluble form, it effortlessly erases the corrosive effects of oxidation, leaving the metal surface renewed and protected. Additionally, its descaling properties dissolve mineral deposits, eradicating the unsightly build up and restoring the metal’s smooth texture and captivating luster.
Industrial Applications
Sodium acetate finds applications in various industries due to its diverse properties. Some of the applications are: –

Textile Industries
Sodium acetate is widely used in the textile industry for multiple purposes. It serves as a neutralizing agent for sulphuric acid waste streams, aiding in waste management and environmental compliance. Additionally, sodium acetate acts as a photoresist when combined with aniline dyes, enabling patterned coating in photolithography and photoengraving processes. It is also utilized as a pickling agent in chrome tanning, enhancing the quality of leather production. Sodium acetate is further employed in textile dyeing, where its combination with aniline dyes derived from coal tar extracts helps achieve vibrant and long-lasting colours.

Synthetic Rubber Industry
In the synthetic rubber industry, sodium acetate plays a crucial role by impeding the vulcanization of chloroprene. This control over the vulcanization process is essential for achieving desired properties and quality in synthetic rubber production.

Cotton Processing Industry
Sodium acetate is utilized in the cotton processing industry, specifically in the production of disposable cotton pads. Its ability to eliminate static electricity build up ensures the efficient and high-quality manufacturing of cotton pads.

Food Industry
Sodium acetate in food finds applications in the industry, where it serves various purposes. It is commonly added to food as a seasoning, often in the form of sodium diacetate, which is a combination of sodium acetate and acetic acid (sodium acetate E number: E262). This addition imparts a salt and vinegar flavour to popular snacks like potato chips. In food production, sodium acetate is used to lower or stabilize the pH of products, ensuring consistent quality and taste. Its acidic properties also inhibit bacterial growth, making it an effective preservative. Sodium acetate is particularly employed in pickling processes to enhance flavours and preserve foods in large batch quantities.

Chemical Industries
Within the chemical industry, sodium acetate plays a significant role in various applications. It is utilized as a chemical compound and has diverse uses. Sodium acetate is commonly employed as a buffering agent, helping to maintain and stabilize the pH of solutions in chemical processes. It can also serve as a catalyst or reactant in certain chemical reactions, facilitating the production of desired compounds. Furthermore, sodium acetate is utilized in the formulation of specialty chemicals, such as those used in the pharmaceutical, cosmetic, and personal care industries. Its versatile properties make it a valuable ingredient in chemical formulations, contributing to the development of a wide range of products.
In summary, sodium diacetate is a versatile compound with numerous applications in various industries. It is used as a preservative, pH regulator, buffering agent, and mold inhibitor, among other uses. Its wide range of applications makes it an important compound in the production of many products, from food and pharmaceuticals to industrial equipment and chemicals.
Sodium Acetate Uses
Sodium acetate has several uses including:
- Concrete Sealant: Sodium acetate is used as a concrete sealant to mitigate water damage. It penetrates the surface of concrete, sealing it against weather elements and preventing water permeation. This application is cost-effective and environmentally benign compared to epoxy alternatives.
- pH Buffer: A solution of sodium acetate and acetic acid acts as a buffer, maintaining a relatively constant pH level. Sodium acetate buffer is particularly useful in biochemical applications where pH-dependent reactions occur within a mildly acidic range (pH 4-6).
- Dialysis Solution: Sodium acetate is employed in dialysis solutions, aiding in the process of blood filtration and waste removal in patients undergoing dialysis.
- Catalysis in DNA Isolation: Sodium acetate serves as a catalyst in the precipitation yield of DNA during the isolation process. It is widely used as a reagent in molecular biology and biochemistry.
- Electrolyte Replenishment: Intravenous transfusions of sodium acetate are recommended for patients undergoing dialysis to replenish electrolyte levels. It is particularly useful for stabilizing falling sodium levels in Hyponatremia patients.
- Heating Pads and Hand Warmers: Sodium acetate is utilized in reusable heating pads and hand warmers. These products rely on a supersaturated solution of sodium acetate and water surrounding a metal disc to generate heat.
Metal Surface Cleaner: Sodium acetate effectively removes impurities and contaminants such as rust, stains, or scale from metal surfaces. It is commonly used as a cleaning agent for metal objects.
Where Can I Buy Sodium Acetate?
If you are in need of sodium acetate for your business don’t worry, Vinipul Chemicals Pvt. Ltd. is your one stop-shop. We stand out as a prominent and trusted entity in the industry, specializing in the manufacturing, supplying, exporting, stocking, and dealing of Sodium Acetate. With a comprehensive range of chemical products, our offerings cater to various sectors, including Chemical Industries, Food Industry, Textile Industry, Rubber Industry, and Cotton Processing Industry. We serve clients across India, as well as destinations in the Indian Subcontinent, East Asia, South East Asia, and the Middle East.
At Vinipul Chemicals Pvt. Ltd., our primary focus is customer satisfaction, and we achieve this by delivering chemical products of the highest quality. Our manufacturing processes adhere to rigorous standards and stringent quality control measures to ensure that our Sodium Acetate and other chemical compounds meet the highest industry standards. We are committed to providing reliable and consistent products that align with the specific requirements and expectations of our esteemed customers.
FAQ's
Sodium Acetate – Pure or Technical Grade – is available in both crystal and anhydrous forms. Subtypes include Sodium Acetate and Sodium Diacetate.
Sodium Acetate is widely used in the textile industry, food processing, and as an intermediate in chemical manufacturing.
It offers excellent solubility, a stable shelf life, and multifunctional use across food, textile, and chemical sectors. It also acts as a buffering agent and rust remover.
Yes, our Sodium Acetate meets REACH, RoHS, GOTS, ISO, and food-grade standards.
Sodium Acetate has a shelf life of 2 years from the date of manufacturing. Store in a cool, dry place, away from sunlight and moisture.
No, it is not hazardous. However, wearing protective gloves and avoiding inhalation of dust is advisable.
We offer 25 kg and 50 kg packaging options in HDPE bags and drums
Yes, we provide both neutral and branded labeling based on client preferences.
Most orders are dispatched within 1 to 2 working days.
Yes, we serve PAN India and export globally.
Yes, all regulatory documents are included as per our standard operating procedure.
Yes, we provide detailed QC reports for each production batch.
Yes, we offer samples for quality evaluation prior to bulk purchase.
Yes, third-party testing can be arranged upon request.
The minimum order quantity is 25 kilograms.
Yes, we offer trial pricing for first-time buyers.
We quote prices based on customer preference—EXW, FOB, or CIF.
Yes, we offer both GST-inclusive and export invoices as required.
Yes, volume-based discounts are available and product-specific.
Yes, we replaced an imported product for a food company with better yield and cost-effectiveness.
Our customers cite high purity, consistent results, snow-white appearance, and fast delivery.
We solve quality inconsistencies, late dispatches, and lack of proper documentation—issues often reported with other suppliers.
We ensure prompt feedback collection, new order coordination, dispatch tracking, and complaint resolution.
While we can’t disclose names, our products are trusted by leading Indian and export-based companies.
Yes, we are ISO-certified and follow GMP-aligned manufacturing practices.
We deliver high purity at competitive pricing, backed by reliable logistics and full documentation.
New buyers often request credit terms. We offer credit after 3 successful prepaid orders or with verifiable references.
Yes, due to superior product quality, faster delivery, and responsive service.
Sodium Acetate is a salt, derived from a weak acid (acetic acid) and a strong base (sodium hydroxide).
It is also called sodium ethanoate or acetic acid, sodium salt.
A 60mm solution of sodium acetate typically has a pH around 4.6.
It is a strong electrolyte but is chemically neutral (not a strong acid or base).
Yes, it is highly water-soluble, making it easy to use in a wide range of applications.
Yes, it is effective in removing stains and rust from metal surfaces.
Yes, it is generally recognized as safe (GRAS) when used within prescribed limits in food.
Store in sealed containers, away from moisture and light, in a cool, dry area.
Available in 25 kg / 50 kg HDPE bags, drums, or custom packs as per client requirements.
You can purchase from Vinipul Chemicals Pvt. Ltd., a trusted manufacturer, supplier, and exporter of premium Sodium Acetate.
Market Area
We supply and exports Sodium Acetate in all parts of the world such as
Sodium Acetate in Africa Countries
South Africa , Nigeria, Kenya, Ghana, Ethiopia, Tanzania, Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic (CAR), Chad, Comoros, Democratic Republic of the Congo, Cote d’Ivoire, Djibouti, Egypt, Equatorial Guinea, Eritrea, Gabon, Gambia, Guinea, Guinea-Bissau, Lesotho, Liberia, Libya, Madagascar, Malawi, Mali, Mauritania, Mauritius, Morocco, Mozambique, Namibia, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, Somalia, South Sudan, Sudan, Swaziland, Togo, Tunisia, Uganda, Zambia, Zimbabwe.
Sodium Acetate in Gulf Countries
Oman, Qatar, Kuwait, Saudi Arabia, Dubai, Bahrain, Iran, United Arab Emirates
Sodium Acetate in Asia Countries
Afghanistan, Armenia, Azerbaijan, Bahrain, Bangladesh, Bhutan, Brunei, Cambodia, China, Cyprus, Georgia, India, Indonesia, Iran, Iraq, Israel, Japan, Jordan, Kazakhstan, Kuwait, Kyrgyzstan, Laos, Lebanon, Malaysia, Maldives, Mongolia, Myanmar (Burma), Nepal, North Korea, Oman, Pakistan, Palestine, Philippines, Qatar, Russia, Saudi Arabia, Singapore, South Korea, Sri Lanka, Syria, Taiwan, Tajikistan, Thailand, Timor-Leste, Turkey, Turkmenistan, United Arab Emirates (UAE), Uzbekistan, Vietnam, Yemen
We supply Sodium Acetate in all parts of India.
Andhra Pradesh, Arunachal Pradesh, Assam, Bihar, Chhattisgarh, Goa, Gujarat, Haryana, Himachal Pradesh, Jammu & Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Manipur, Meghalaya, Mizoram, Nagaland, Odisha, Punjab, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Uttarakhand, Uttar Pradesh and West Bengal.
Note: – Please be advised that the information contained in this document is intended for illustrative purposes only. Due to variations in product grade, applications, industries, or uses, the accuracy of the information provided cannot be guaranteed. © Copyright 2023 © Vinipul Chemicals All Rights Reserved (Terms of Use). Reproduction of any material from this site is strictly prohibited without permission. Vinipul Chemicals products are exclusively sold through the company’s website. For precise product specifications and requirements, as well as advice on which products are best suited for your specific application needs, please contact us at business@vinipulchemicals.com Use Terms | Privacy.


